Cardiac Development & Gene Regulation
Congenital heart disease (CHD) is the most prevalent birth defect, occurring in about 1% of all live births. However, in over half of these cases, the genetic cause remains unidentified. Despite notable progress in biomedical research and surgical interventions, CHD persists as a leading contributor to morbidity and mortality across both pediatric and adult populations. While many cases stem from disturbances in developmental gene networks, our limited understanding of the fundamental gene regulatory mechanisms restricts the interpretation of non-coding mutations identified through whole-genome sequencing, often hindering comprehension of CHD etiologies.
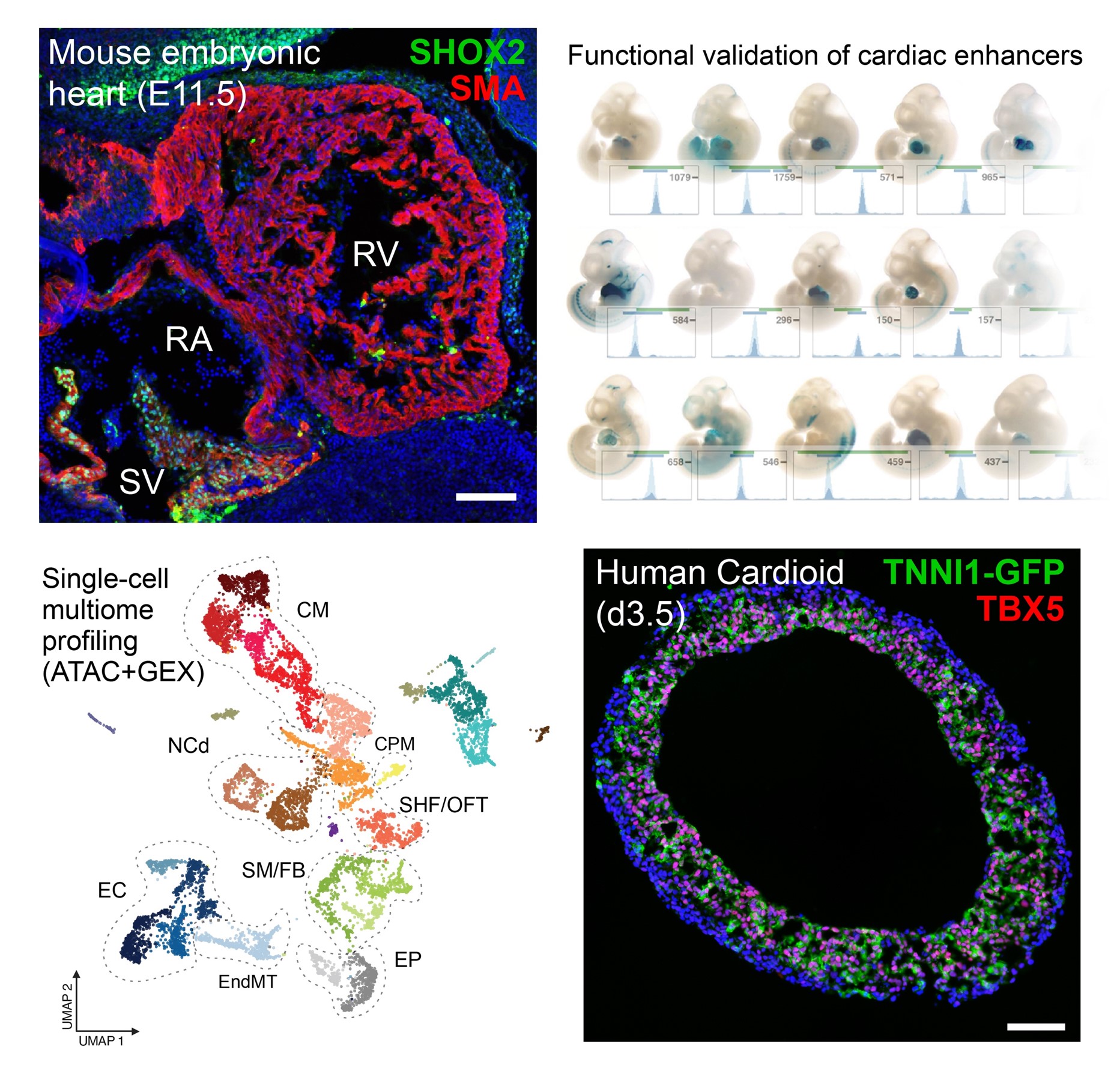
The Osterwalder Lab combines functional genomics with reverse molecular genetics to explore the gene regulatory architecture that orchestrates formation of the four-chambered heart during embryogenesis. Their research focuses on DNA-encoded “transcriptional enhancers”, key regulatory elements that precisely control when, where, and how strongly genes are turned on or off in specific cardiac cell types. Notably, these enhancers can regulate genes from great distances, sometimes located as far as a million base pairs away from their target genes in the genome.
Mutations or disruptions in genomic enhancers are strongly associated with developmental malformations. However, the mechanistic contribution of cardiac enhancers to heart malformations or disease remains poorly understood due to limited functional mapping. To address this gap, the Osterwalder lab integrates single-cell multiomic profiling, chromatin conformation capture, molecular analyses, and CRISPR/Cas9 genome editing, utilizing mouse embryos and human induced pluripotent stem cell (hiPSC)-derived cardiac organoids as state-of-the-art model systems. This combined strategy seeks to unravel the complex enhancer networks that regulate heart development and to identify key enhancer regions essential for preventing cardiac malformations and arrhythmias. Translating these mechanistic insights is crucial for interpreting non-coding genetic variants linked to heart disease to enhance genetic diagnosis in patients and to lay the foundation for future regenerative therapies focused on epigenome or base editing.
Contact
Marco Osterwalder, Ph.D.
Research Group Leader
University of Bern
Department for Biomedical Research (DBMR)
Murtenstrasse 24
3008 Bern
Phone: +41 (0)31 684 04 13
Email: marco.osterwalder@unibe.ch
ORCID iD: 0000-0002-1969-2313
Google Scholar: https://scholar.google.com/citations?user=av6TeC8AAAAJ&hl=en
Group website: https://www.osterwalderlab.com