Ongoing projects

SNF Sinergia Project 1: Xeno2Cure - advanced engineering and testing of organ donor pigs (PI: Robert Rieben, Co-PIs: Jörg Seebach, Wolf Eckhard)
The overall aim of this project is to generate pigs with multiple genetic modifications available for testing in experimental pig-to-baboon and later on in clinical pig-to-human xenotransplantation. In order to achieve this, we propose to re-design genetically modified donor pigs ‘from scratch’ and assess the functional consequences of the combined genetic modifications on a cellular level in vitro before pigs are produced from these modified cells. Pigs will then be bred based on the genetic modifications which were proven in vitro to have the best functional phenotype. Finally, ex vivo perfusion studies of limbs of these animals with human blood will be performed to further assess the suitability of the respective genetic modifications in potential xenograft organ donor pigs.
This project combines animal genetics and biotechnology with immunology, in particular humoral and cellular innate immunity, but also direct T cell activation by endothelial cells (EC). In addition, biomedical engineering tools like microfluidic techniques for 3D culture of EC under pulsatile flow will be used. Only this synergistic combination of disciplines and approaches will allow for the breakthrough, which is necessary to functionally understand and correctly design in a hypothesis-driven, bottom-up approach the optimal set of genetic modifications required to make porcine organs compatible enough with human physiology and the human immune system to allow clinical xenotransplantation.
SNF Project 2: Endothelial Cell Protection in Ischemia/Reperfusion Injury: Investigating the Role of the Glycocalyx and the Plasma Cascade Systems (PI: Robert Rieben)
Ischemia/ Reperfusion injury (IRI) refers to tissue damage that can occur after restoration of blood flow in a previously ischemic area, examples include myocardial infarction or also organ transplantation. A key event in IRI is activation of endothelial cells (EC) leading to a dysregulation of plasma cascade systems and vascular leakage. Both the complement system and the endothelial glycocalyx are important players in vascular homeostasis and complement activation as well as glycocalyx damage have been observed in IRI. We want to understand how EC and their glycocalyx contribute to the maintenance of vascular integrity which could help identify new therapeutic targets in the field of IRI. To investigate this, we use a unique 3D microfluidic model established in our lab which consists of round-section, vessel-like microfluidic channels containing EC exposed to pulsatile flow to reproduce physiological conditions in vitro. This project provides valuable fundamental research that is much needed in the field of IRI.
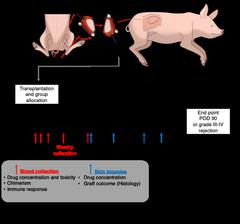
SNF Project 3: Site-specific immunosuppression for long-term maintenance of vascularized composite allotransplantation: validation of efficacy and safety in a clinically relevant large animal model (PI: Radu Olariu, Co-Pi: Robert Rieben)
Vascularized composite allotransplantation (VCA), such as hand-transplantation, has the potential to restore esthetic and function in patient that suffered severe injuries. However, adverse effects of chronic high-dose immunosuppression regimens strongly limit the access to procedures in the clinic. Unlike most solid organ transplants, VCA offer unique opportunities for delivery of immunosuppressive medications directly to the graft. Recently, we developed enzyme-responsive tacrolimus-encapsulated hydrogels (TGMS-TAC) as an “on demand” drug delivery system (DDS) that titrates the amount of drug released in response to inflammation. We proved that a single injection of TGMS-TAC increased graft survival in a rat hind limb transplant model. Moreover, we investigated the long-term outcomes using the same model demonstrating long-term effectiveness and improved pharmacological, toxicological and immunological profile of site-specific immunosuppression. We have also developed an in situ forming implant loaded with rapamycin (Rapa-ISFI) demonstrating that this DDS successfully promotes immunoregulation trough mixed chimerism and donor-specific Treg induction and establishment of peripheral tolerance and long-term acceptance of VCA. Overall, in this project we will provide clear-cut data on the feasibility and validity of DDS-based approaches in a model relevant to clinical human VCA, filling the gap between basic research developments and clinical application and paving the way for human clinical trial.
SNF Project 4: Focus research CoVasc-consortium (PI: Yvonng Döring, Co-PIs: Robert Rieben, Britta Engelhardt, Nadia Mercader)
Severe Acute Respiratory Syndrome-CoronaVirus 2 (SARS-CoV-2) is the causative agent of COronavirus DIsease 2019 (COVID-19), whereof the complete pathogenesis still remains to be elucidated. Our aim is to investigate the influence of SARS-CoV-2 infection on the cardiovascular system of humans and to analyze how the complement system plays a role in the pathogenesis of COVID-19. To analyze this, we mainly focus on cells of the cardiovascular system, like micro- and macrovascular endothelial cells, pericytes and smooth muscle cells. Our approach is to analyze these cells using a microfluidic system a 3D vessel-like structure that allows a pulsatile flow condition. Cells are infected using Vesicular Stomatitis Virus (VSV)-derived pseudoparticles that express the spike protein of SARS-CoV-2 or alternatively, directly infected with iSARS-CoV-2 in our biosafety laboratory (BSL)-3 facility
In addition, we will investigate how these cells respond to viral infection by stimulation using various Toll-like receptor (TLR) agonists like Poly (I:C) or Lipopolysaccharide (LPS). Shedding of the glycocalyx, secretion of cytokines and upregulation of genes will be then analyzed.
News articles:
Hier infizieren Forscher menschliche Zellen absichtlich mit dem Coronavirus
Berner Wissenschaftler nehmen das Virus ins Visier
Melle Holwerda, 30, über Covid-19-Forschung im Hochsicherheitslabor: So sehe ich das. Schweizer Familie 25/2021
DoD Project: In Vivo and Ex Vivo Models to Study Ischemia / Reperfusion Injury, Endothelial Cell Protection and Limb Preservation in a Prolonged Field Care Scenario (PI: Robert Rieben)
This project aims to study and prevent ischemia/reperfusion (I/R) injury in amputated extremities, with the clinical goal to extend the duration from traumatic amputation to successful surgical replantation. We will focus on the limited intervention capacity at the point of injury that may dictate the use of a tourniquet to prevent fatal blood loos. A delay of several hours or even days prior to revascularization or replantation may lead to severe I/R injury. Extensive muscle tissue damage will be the local consequence in the affected extremity, but severe I/R injury may also lead to systemic inflammatory response syndrome (SIRS) or even multiorgan failure (MOF). The models to be developed will therefore be used to provide guidance in the management of I/R injury and to test technical feasibility and clinical efficacy of promising therapeutic interventions. The final, clinical goal is to find ways to prevent I/R injury by using endothelial cell protection and extracorporeal perfusion. An acute setting with extended anesthesia duration in pigs will be used, combined with state-of-the art analysis of innate immune activation, including complement and coagulation, as well as assessment of endothelial cell activation with a particular focus on the glycocalyx.

Innosuisse Project: Developing a next-generation targeted anti-fibrotic therapy for heart failure in a porcine model (PI: Robert Rieben)
Acute myocardial infarction (AMI) is the most severe manifestation of coronary artery disease. One of the treatment for AMI consists in percutaneous coronary intervention, aiming at restore tissue perfusion. However, as a consequence, ischemia/reperfusion (I/R) injury occurs, possibly increasing the myocardial necrotic area. During post-infarction tissue remodelling, fibrotic tissue will develop. Not contributing to the pump function of the heart, it will lead to heart failure development.
The aim of this trial is to test a chemical compound targeting fibrosis by inhibiting the long non-coding RNA (lncRNA). Wisper acts binding to a pleiotropic master regulator of fibrosis, TIA1 cytotoxic granule associated RNA binding protein-like 1 (TIAR), stimulating its nuclear translocation and promotion of pro-fibrotic target gene transcription, specifically in the heart. Previous studies in vivo murine model of myocardial infarction, showed that inhibition of Wisper using an antisense oligonucleotide reduced cardiac fibrosis by 70% (Micheletti et al., 2017).
Antisense oligonucleotide (ASO) therapeutics are injectable synthetic single-stranded deoxynucleotide polymers that are designed complementary to an RNA or DNA sequence. In this trial we aim to apply two well-established ASO chemistry platforms on top of a standard phosphorothioate-modified backbone in Göttingen minipigs undergoing AMI. The therapeutic approach that will be explored in this study could be the first disease-modifying drug for heart failure directly addressing cardiac fibrosis in a large animal model. Moreover, due to the paucity of information about pain in minipigs and especially visceral pain, despite their widespread use in cardiovascular biomedical research, a satellite study will be conducted with the aim at evaluating pain in minipigs undergoing AMI.

CSLB Project: In Vitro Drug Screening on Endothelial Cells in a 3D Microfluidic Model (PI: Robert Rieben)
During the development of new drugs, it is necessary to perform drug screening to test the efficiency or side effects of the drug candidate. This can be performed in vivo on animal models but recent advances in the field of in vitro techniques can now offer interesting alternatives. One of these is our unique 3D in vitro microfluidic model in which vascular endothelial cells (EC) are grown in round-section, vessel-like microfluidic channels. This allows to test different drugs acting on EC but also to check for unwanted toxicity, pro-inflammatory or pro-coagulant activity of new drug candidates towards endothelial cells. The 3D microfluidic model allows for easy imaging but can also be used to extract RNA or proteins for qPCR, sequencing or western blot applications. In a collaborative effort with CSL Behring this system is currently being further optimized to provide higher throughput as well as consistency across experimental set-ups.
CSLB Project: The role of citrullination in ischemia reperfusion injury (PI: Nicoletta Sorvillo)
This project is funded by CSL Research Acceleration initiative grant and is led by Dr. Nicoletta Sorvillo, who’s expertise in neutrophils extracellular traps (NETs) and PAD4-mediated citrullination has allowed the identification of citrullinated plasma proteins in thrombotic disorders. Currently, Valentina Zollet under the supervision of Dr. Sorvillo, is analyzing the role of citrullination in ischemia reperfusion injury (IRI) with the aim to identify new therapeutic targets and biomarkers. IRI is a pathological inflammatory process that occurs upon reperfusion of a tissue after interruption of blood supply. It occurs during several pathological conditions such as stroke, myocardial infarction or after surgery and transplantation. Despite the advances in our understanding of IRI, the molecular mechanisms leading to IRI are still largely unknown. Since IRI is a critical medical condition, gaining insight into the molecular pathways involved in its complex pathophysiology is necessary for developing therapies to limit the devastating health and economic burdens imposed by this disorder.
Neutrophils, migrate to the injured tissue during inflammation and release, upon activation, NETs, a web-like structure composed of decondensed chromatin and neutrophil proteins. NETs participate to IRI by damaging the endothelium and activating inflammatory and coagulation pathways. Inhibition of NET formation or degradation of NETs in animal models of IRI reduces tissue damage. Peptidyl arginine deiminase 4 (PAD4) is liberated in the extracellular milieu upon NET formation. Although NETs and serine proteases are involved in IRI, the impact of PAD4 and citrullination is unknown. Currently, we are determining the effect of PAD4 during IRI and identifying citrullinated proteins using a large translational pig model of IRI. Our studies will allow the discovery of novel therapeutic targets and contribute to the identification of novel early injury biomarkers in IRI, e.g. in transplantation, myocardial infarction, or stroke.
CSLB Project: Prevention of ischemia/reperfusion injury by C1 INH: Assessment of reperfusion injury in total knee arthroplasty (PI: Robert Rieben)
Total knee arthroplasty (TKA) is an elective and effective surgical procedure to overcome the chronic pain and mobility limitation due to osteoarthritis. Intentional ischemia is usually required to produce a bloodless surgical field and to reduce blood loss during the surgery. Tourniquet use leads to an ischemia of the knee and lower limb ranging from 30 to 120 minutes. It is known that reperfusion of an organ or tissue which were ischemic for a prolonged period of time can lead to ischemia/reperfusion (I/R) injury. I/R injury is an inflammatory reaction of the tissue, probably caused by expression of ‘danger’ signals on the ischemic endothelium, and involves activation of the endothelium, the plasma cascades and the innate immune system. So far, however, the role of endothelial cell activation, innate immunity and activation of the plasma cascade systems in I/R injury in the context of tourniquet use in orthopedic surgery has not been investigated in detail. In clinical practice, postoperative edema formation on the lower limb after TKA is often thought to be caused by surgical trauma rather than I/R injury. However, it has been described that a longer ischemia time in TKA leads to more severe postoperative pain and edema formation, and the link to I/R injury has been made in this study. Currently we are assessing the role of activation of the endothelium and the plasma cascades in I/R injury after TKA in order to establish a basis for a possible clinical study with C1-inhibitor.